In this post
The nuclei of some atoms are unstable. These atoms decay and emit radiation in the form of alpha or beta particles or gamma rays. This radiation can be detected using a device called a Geiger-counter. As well as emitting radiation, this process of radioactive decay produces energy on a relatively small scale. Nuclear reactions such as the splitting or fusing together of atomic nuclei also produce large amounts of energy.
Nuclear fission
The splitting of unstable atoms into smaller atoms is a type of nuclear reaction known as nuclear fission. The process of nuclear fission produces large amounts of energy which can be used to generate electricity in nuclear power stations. The energy produced in the process of nuclear fission is used to heat up the water and generate steam.
This steam then travels upwards and turns a turbine. The turbine spins the generator and electricity is generated. Electricity travels through the step-up transformer where it is converted to the correct voltage to allow transmission across the national grid.
The fuel used in most nuclear power stations is uranium-235 (U-235). In the nuclear reactor of the power station, an atom of U-235 collides with a neutron which is absorbed into the nucleus. By absorbing the neutron, the nucleus of the U-235 becomes unstable and splits. This splitting is known as nuclear fission. For every atom of U-235 two radioactive daughter nuclei and two or three neutrons are produced. An atom of U-235 absorbs the neutron causing it to decay to krypton-91 and barium-143, along with two neutrons. The process of fission produces large amounts of energy. This energy is transferred to the fission products as kinetic energy allowing them to move quickly.
The neutrons produced as a result of the fission process then go on to collide with and be absorbed by other U-235 atoms causing them to become unstable. These unstable nuclei then split and release more neutrons. This process of nuclear fission can continue as long as the neutrons are being produced, and is known and a chain reaction. A typical nuclear reactor is shown in the diagram below
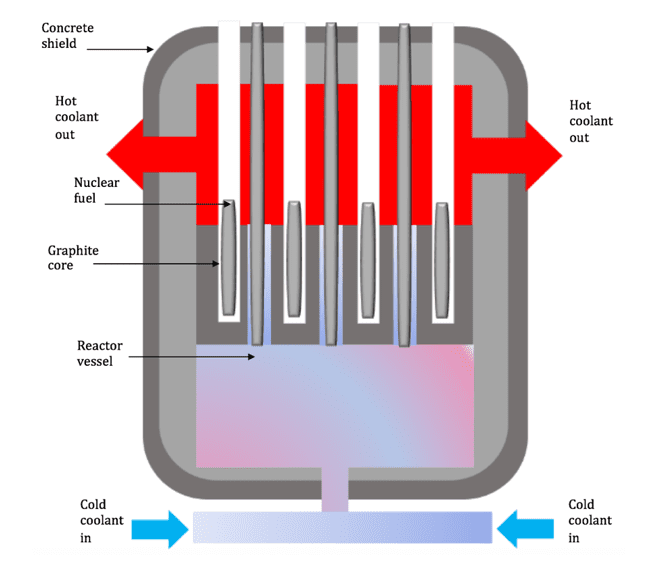
If all of the uranium nuclei absorb all neutrons, the chain reaction can spiral out of control. The large amount of energy produced by the uncontrolled chain reaction could potentially cause an explosion. An explosion would cause the release of dangerous radioactive material into the environment and therefore must be avoided.
The amount of energy produced by nuclear fission can be controlled by controlling the rate of the chain reaction occurring in the reactor. The rate of fission can be controlled using control rods. These control rods are made of boron and are able to absorb some of the neutrons produced during nuclear fission. This absorption means that fewer neutrons are available to collide with and split the uranium nuclei. The control rods are raised to increase and lowered to decrease the number of free neutrons. When the control rods are lowered into the reactor more neutrons are absorbed and the rate of nuclear fission is decreased. When the control rods are raised out of the nuclear reactor, no neutrons are absorbed and the rate of fission increases.
If the nuclei travel too quickly, they may simply move past the U-235 atoms rather than colliding with them. The moderator in a nuclear reactor contains the fuel rod and is usually made of graphite or heavy water. The moderator is used to slow down the neutrons produced, increasing the likelihood of them colliding with the U-235 nuclei. The shielding used around the nuclear reactor is usually made of thick concrete. This is used to absorb neutrons and ionising radiation preventing it from escaping into the surrounding environment and thus protecting people from serious danger. Ionising radiation in the reactor itself is prevented from escaping by steel and thick concrete walls.
Nuclear fission causes unstable atoms to split producing two radioactive daughter nuclei and two or three neutrons. Fusion is the word used to describe things coming together.
Nuclear fusion
Nuclear fusion is the opposite to nuclear fission. In the nuclear fusion process two smaller atomic nuclei collide and fuse together to form a larger nucleus. This results in the loss of mass. The mass of the larger nucleus produced is lower than the combined mass of the two smaller nuclei. This is because some of the mass is converted into energy. This energy is released in the form of radiation.
The amount of energy produced from nuclear fusion is much greater than that produced from nuclear fission.
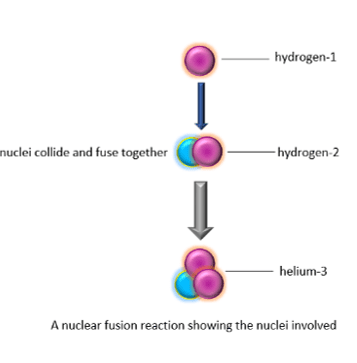
The Sun and other stars use nuclear fusion to release energy. The sequence of nuclear fusion reactions in a star is complex, but, overall, hydrogen nuclei join to form helium nuclei and energy as shown below:
![Rendered by QuickLaTeX.com \[ \ce{^{1}_{1}H} + \ce{^{2}_{1}H}\rightarrow \ce{^{3}_{2}H +\text{energy}} \]](https://env-onlinelearningcollege-gpclone.kinsta.cloud/wp-content/ql-cache/quicklatex.com-e6232a359f7f7f65f7747ee157aece5d_l3.png)
Nuclear fusion involves a deuterium and a tritium nucleus colliding and being forced together. Both nuclei are positively charged due to the protons contained which repel each other. This is known as electrostatic repulsion. The nuclei need to be moving very fast so that they can get close and collide. The energy needed to provide this kinetic energy and overcome the repulsive forces is very high. The hotter a molecule is, the faster it will move and the more likely it is to collide. The temperatures and pressures required for nuclear fusion to occur are very high.
Therefore, the temperature and pressure needed in a nuclear fusion reactor would be extremely high. It is very difficult and expensive to build reactors which are capable of providing these temperatures and pressures. Currently we have no nuclear reactors which are capable of using nuclear fusion to generate energy.



