In this post
Isomerism is defined as the existence of molecules with the same molecular formula but different arrangement of atoms in space. Structural isomers are molecules with the same molecular formula but a different structural formula. This means that the order in which the atoms are arranged in each isomer differs from the others.
Structural isomerism of alkanes
Alkanes which contain four or more carbon atoms have structural isomers.
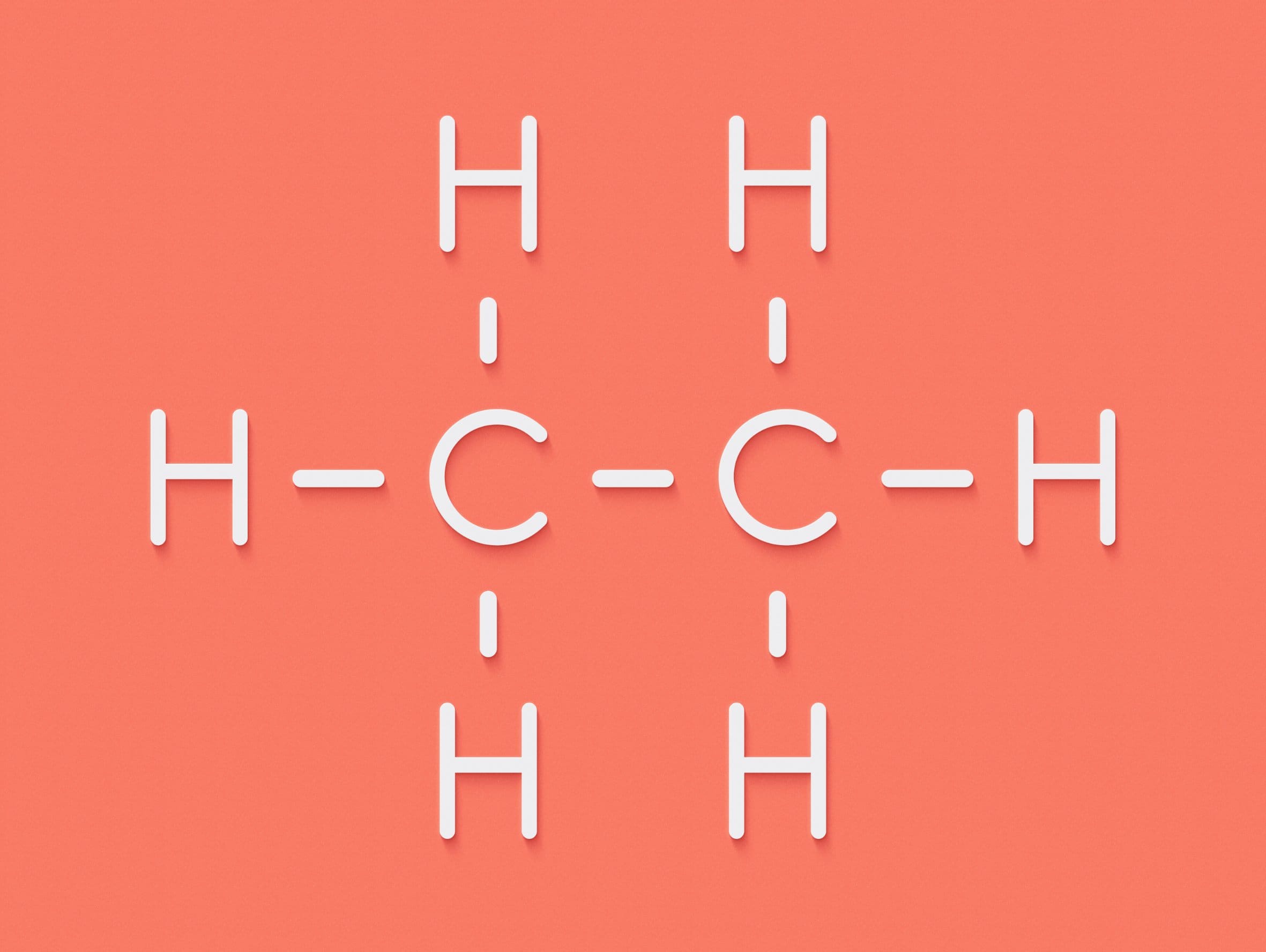
For example, an alkane with a molecular formula of C4H10 could have either of the structural and displayed formulae shown below:
| Structural formula: | CH3CH2CH2CH3 | CH3CH(CH3)CH3 |
| Displayed formula: |  | 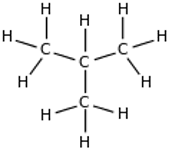 |
| Name | butane | 2-methylpropane |
Butane and 2-methylpropane share the same molecular formulae, but as their structural formulae and arrangement of atoms differ, we can say that they are structurally isomers. Pentane also demonstrates structural isomerism. The molecular formula of pentane is C5H12 and this molecule exists as three different structural isomers:
| Pentane | CH3CH2CH2CH2CH3 | 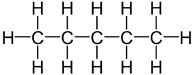 |
| 2-methylbutane | CH3CH(CH3)CH2CH3 | 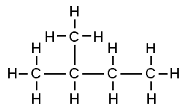 |
| 2,2-dimethylpropane | CH3C(CH3)( CH3)CH3 | 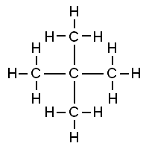 |
Structural isomerism of alkenes
The position of the carbon-carbon double bond in alkenes can vary if there are four or more carbon atoms in the chain. Ethene only contains two carbon atoms so the double bond only exists in one place. Propene contains three carbon atoms, so the double bond can only exist between the first and second carbon atoms.
Alkenes which contain four or more carbon atoms have structural formulae. The alkene butane has the molecular formula C4H8. Two possible structural isomers for butene are but-1-ene where the double bond is found between the first and second carbon atoms or but-2-ene where the double bond is found between the second and third carbon atoms.
A third isomer of C4H8 is 2-methylpropene where there is a double bond between the first and second carbon atom and a methyl side chain is attached to the second carbon atom.