In this post
A catalyst is a substance which increases the rate of a chemical reaction but is chemically unchanged at the end of the reaction. Different catalysts are used in different reactions. Some of the most common catalysts used in industries are shown below:
| Catalyst | Reaction that is catalysed |
| Iron | Making ammonia from nitrogen and hydrogen |
| Platinum | Making nitric acid from ammonia |
| Vanadium (V) oxide | Making sulphuric acid |
Activation energy is the minimum amount of energy required for a reaction to occur. Collisions can only be successful if the reactant particles colliding have energy equal to or greater than the activation energy.
Reaction profile diagrams
Reaction profile diagrams are similar to the energy level diagrams seen earlier for endothermic and exothermic reactions. They differ in that they also display the activation energy required for the reaction to occur, as can be seen in the example below for an exothermic reaction:
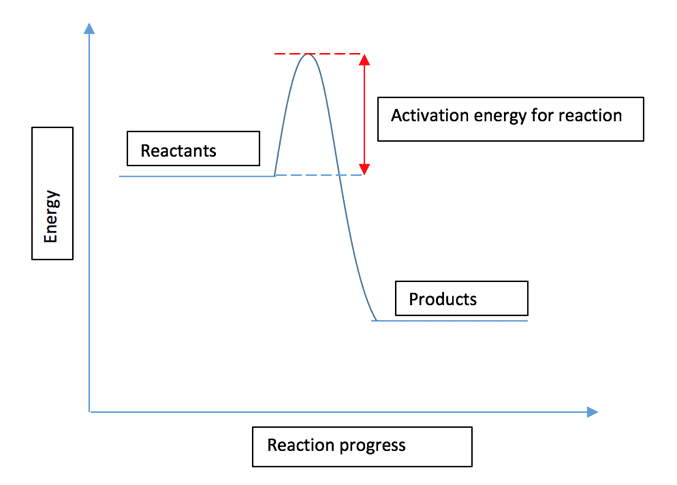
A catalyst works by providing an alternative pathway for the reaction with a lower activation energy. This effect can be shown using the previous reaction profile diagram:
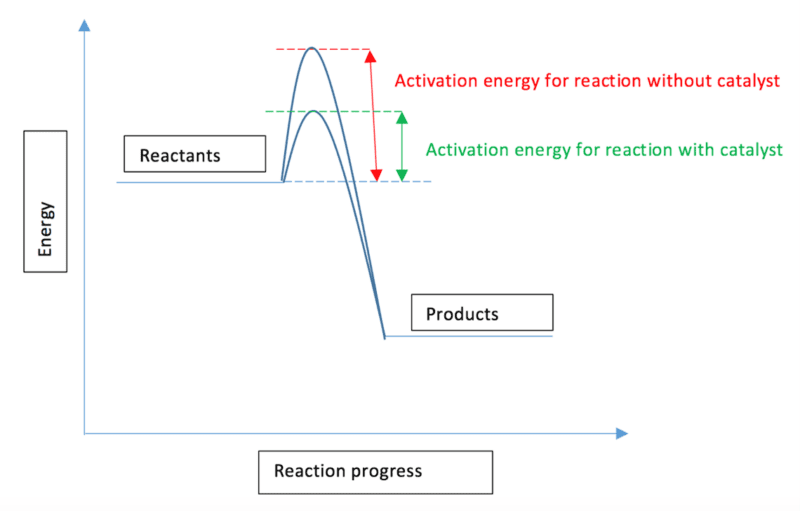
As can be seen on the reaction profile diagram, the activation energy required for the reaction without the catalyst is much higher than that required when a catalyst is used. As the activation energy is lowered, there will be a greater number of particles in the reaction mixture which have energy equal to or greater than the activation energy, and so more successful collisions can occur over a time period. This results in a higher rate of reaction.
The lower the activation required, the lower the temperature needed for the reaction and the lower the amount of energy needed to heat up the mixture. In industry the use of catalysts allows companies to produce their products much faster whilst lowering the costs of the energy required.
Key term
Catalyst – a substance which increases the rate of a reaction but remains chemically unchanged at the end of the reaction.