In this post
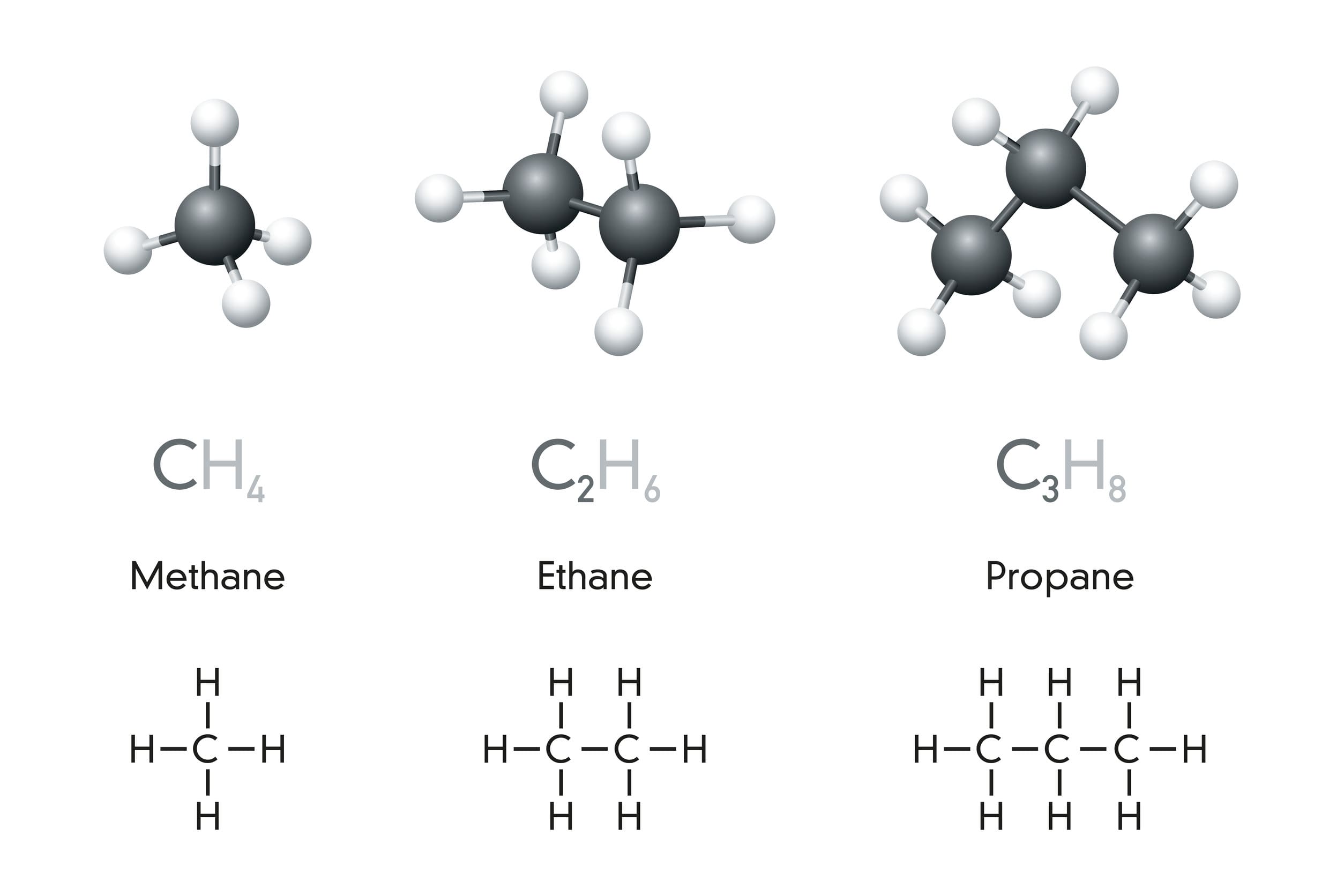
Alkanes are hydrocarbon compounds. This means that they contain only carbon and hydrogen atoms. The molecular formulae of the first two alkanes are CH4 (methane) and C2H6 (ethane). In each of these formulae, the number of hydrogen atoms is equal to double the number of carbon atoms plus two.
Therefore, the general formula for alkanes can be represented as CnH2n+2, where n is the number of carbon atoms in the compound. The general formula can be used to predict the molecular formula of any other alkane. For example, for an alkane containing five carbons, the value of n is 5, meaning that the value of 2n+2 would be 2 x 5 +2 = 12. Therefore, an alkane containing five carbon atoms has a molecular formula of C5H12.
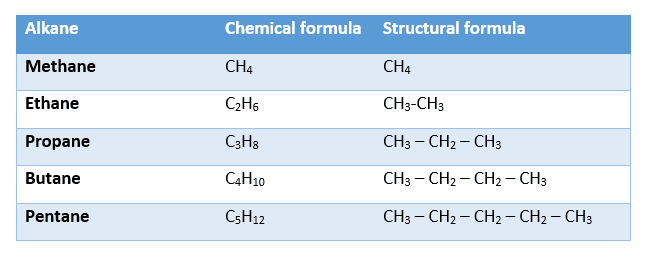
The chemical and structural formulae for the first five alkanes are shown in the table below:
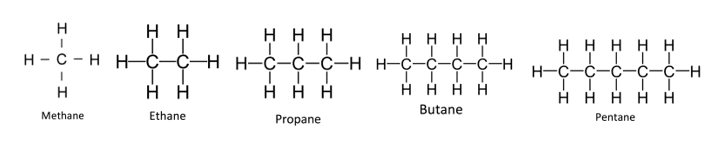
Alkanes are known as saturated hydrocarbons. They only contain single covalent bonds between the atoms. These single covalent bonds are represented using single lines in the displayed formulae of the alkanes. The displayed formulae for the first five alkanes are:
Chemical reactivity
The presence of only single covalent bonds between all atoms makes alkanes relatively unreactive compounds. Energy must be supplied in the form of heat or radiation in order to break these bonds and allow the alkanes to react. As we have already discussed, alkanes will undergo combustion reactions when in the presence of oxygen and a heat source.
Alkanes will also undergo substitution reactions with halogens in a mono-substitution reaction where one of the hydrogen atoms in the alkane is replaced or substituted by a halogen atom such as a chlorine or bromine atom.
These substitution reactions will only take place in the presence of ultraviolet (UV) radiation. When a halogen molecule such as bromine (Br2) or chlorine (Cl2) is exposed to UV radiation, the single covalent bond between the two halogen atoms breaks and two halogen free radicals are formed. A free radical is a particle with an unpaired electron.
One of the single covalent bonds between a carbon and hydrogen atom in the alkane molecule breaks, releasing a hydrogen atom. The carbon atoms then form a covalent bond with the halogen free radical to form a new compound known as a haloalkane and a hydrogen halide.
Methane reacts with chlorine in the presence of UV radiation, to produce chloromethane and hydrogen chloride as shown by the equation below:
![Rendered by QuickLaTeX.com \[\text{Methane} + \text{chlorine} \rightarrow \text{chloromethane} + \text{hydrogen chloride} \]](https://env-onlinelearningcollege-gpclone.kinsta.cloud/wp-content/ql-cache/quicklatex.com-613ef245405ddafb68f25fac868a8b8f_l3.png)
![Rendered by QuickLaTeX.com \[CH_{4(g)} + Cl_{2(g)} \rightarrow CH_3Cl_{(g)} + HCl_{(g)}\]](https://env-onlinelearningcollege-gpclone.kinsta.cloud/wp-content/ql-cache/quicklatex.com-19f0332323a1e0dc8f127aa4153d12e1_l3.png)