In this post
The exchange of nutrients and wastes into and out of a cell is essential to its survival. As we have mentioned previously, cell membranes are selective about the substances that are able to move into and out of cells. Diffusion, osmosis and active transport are three main ways that molecules and ions can move into and out of cells through the membrane.
Diffusion
Diffusion occurs when there is a higher concentration of particles in one place than another. Diffusion is the process in which a higher concentration of particles moves to the area with a lower concentration of particles. For example, the reason you can smell perfume is because the particles diffuse into the air, making their way to your nose.
When diffusion occurs, we say that the particles diffuse down a concentration gradient. Take a look at the diagram below which illustrates carbon dioxide molecules moving into and out of a cell by diffusing down a concentration gradient.
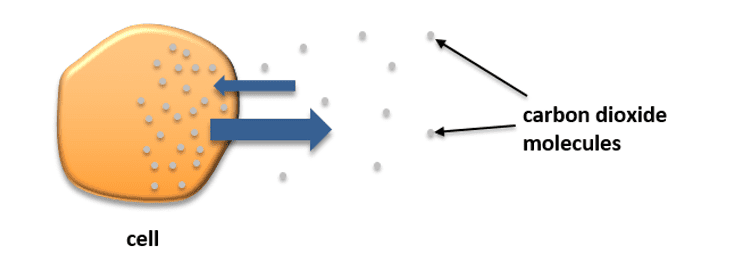

Carbon dioxide is produced in the cell during cell respiration. The process of diffusion is essential for cell respiration. Respiration results in a high concentration of carbon dioxide molecules building up inside the cell. As you can see from the diagram provided, although the molecules diffuse in both directions (into and out of the cell), the net (overall) movement is down the concentration gradient and out of the cell. When the molecules do this, we say that they are attempting to become isotonic. The particles cause diffusion to happen because of their own kinetic energy; they do not require an extra source of energy from respiration.

Active transport
Occassionally, a cell will require a substance that is in small quantities outside of a cell; this means it will work against a concentration gradient (molecules will move across the membrane from a lower to a higher concentration). To achieve this, it will use energy gained from cell respiration. This energy allows for the movement of a liquid from one place to another via a ‘pump’. These ‘pumps’ are large protein molecules, sometimes referred to as carrier proteins, that are located in the cell membrane and pump ions and other molecules into and out of the cell. For example, in humans the cells of the small intestine will absorb glucose via the process of active transport. Take a look at the following diagram for a detailed illustration of active transport.
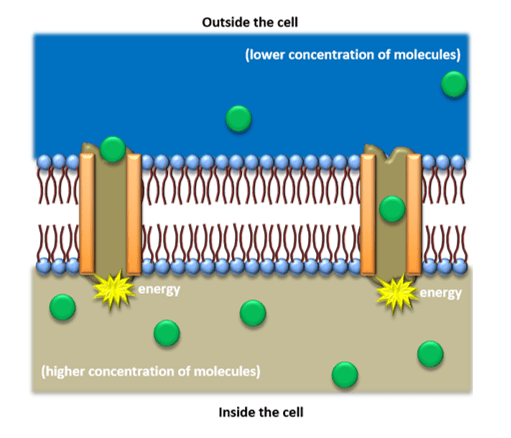
Osmosis
Osmosis is a similar process to diffusion but only involves water molecules. Like diffusion, these water molecules move across a concentration gradient; in other words, from an area of high concentration of water molecules to an area of low concentration (they attempt to become isotonic). However, osmosis can only occur when a cell membrane is partially permeable; in other words, the membrane is permeable to water but not other solutes.
When the concentration of water is isotonic, then the movement of molecules into and out of the cell will be the same. This means that the net exchange of water molecules is zero but they still continue to be exchanged evenly.
Osmosis is a process that is essential for moving water between cells. The membranes of animal and plant cells are partially permeable; this includes the inner membrane surrounding a plant’s vacuole. Although we cannot shown practically the effects of osmosis in cells, we can perform an experiment to illustrate the process of osmosis using Visking tubing.
Practical example
Visking tubing is an artificial partially permeable membrane. It has microscopic holes in its membrane that only allow small molecules through (like water) and not larger molecules (like sugar sucrose); in other words, it is permeable to small molecules like water but not larger molecules – this is why it is known as ‘partially’ permeable. Visking tubing is used as a membrane in kidney dialysis machines and also as an edible casing for sausages. An example of how you might approach a practical experiment to show the effects of osmosis has been provided below.
Equipment required:
- Beaker
- Capillary tube
- Cotton thread
- Sucrose solution
- Visking tubing
- Water
By filling a beaker full of water and placing Visking tubing full of concentrated sucrose solution that is attached to a capillary tube in the water, the effects of osmosis can be shown. As indicated by the red arrow on the illustration, the level of the capillary tube rises when water molecules begin to move from the beaker and through the Visking tubing. This movement of water molecules through the Visking tubing is osmosis.
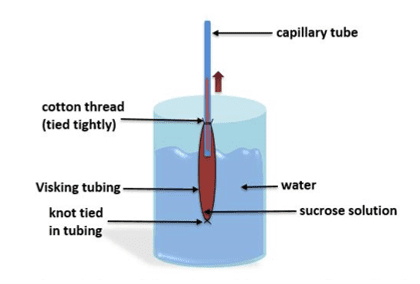
In order to help you fully understand the process of osmosis, a highly magnified illustration has been provided for you below to further illustrate how this process occurs.
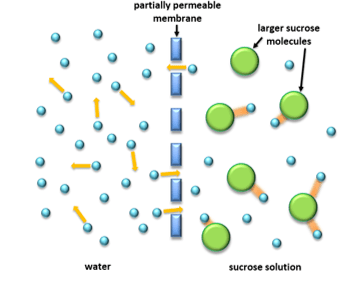
As you can see, the holes in the partially permeable membrane will not allow the larger sucrose molecules to pass through the membrane, however, the water molecules are small enough to pass through freely. The arrows surrounding the blue water molecules on the left indicate their kinetic energy; however, as indicated on the right, the sugar molecules are attracted to the sucrose molecules which reduces their kinetic energy and slows down their movement. As a result, although water molecules are passing through on both sides, there is a greater diffusion of water molecules from the solution of pure water (the more dilute solution) than the sucrose solution (the concentrated solution). In other words, the water molecules on the left are passing through the membrane much more quickly and frequently.
The molecules’ ability to move more freely is known as the water potential. Pure water has the highest water potential as molecules have more kinetic energy and can move around much more freely. In conjunction, the more concentrated a solution, the lower its water potential.
How factors affect the rate of movement of substances into and out of cells
Different factors can affect the rate of substances into and out of cells. In this chapter, we will discuss how surface area to volume ratio, distance, temperature and concentration gradient can affect this rate of movement.
Surface area to volume ratio
Surface area to volume ratio refers to the size of a cell. The bigger the cell, the less surface area to volume ratio. With less surface area to volume ratio diffusion, osmosis and active transport processes become less efficient. The supply rate is the area of the cell’s surface which determines the quantity of oxygen the organism can get. The demand rate is the volume of a cell which determines the quantity of oxygen the organism can use. The ratio for this can be written as:

Let’s think of this in relation to the organisms ‘humans’ and ‘amoeba’ and their absorption of oxygen as an example.
An amoeba is a unicellular organism that is microscopic in size. Like all unicellular organisms, it obtains oxygen through its surface membrane. Due to its small body, its surface is efficient in being able to absorb the oxygen it requires inside of the cell.
However, in regards to humans, the organism is much larger and so its surface area to volume ratio is less. The human body requires much more oxygen than the surface area can provide – than the lungs can effectively release inside the whole body. Thus, we have circulatory systems that carry oxygen from the lungs and around the body, whereas unicellular organisms do not as they don’t require it as their surface area to volume ratio is high.
In other words, the larger the cell, the less surface area it possesses for these processes to work efficiently, and the smaller the cell, the more surface area it possesses for these processes to work efficiently.
Distance
Distance also affects the rate of substances into and out of cells. If the distance from the cell to the particles is far then the time taken for them to reach and be absorbed by the cell’s membrane/point of diffusion is much longer.
Temperature
Kinetic energy increases in particles when a substance is exposed to higher temperatures. This results in the rate of diffusion, osmosis and active transport increasing as the rate of movement of the particles is faster. Remember that active transport is an ‘active’ process which uses energy from cell respiration to move substances into and out of cells. However, the reason why an increase in temperature speeds up the rate of active transport is that the movement of particles to the active site is quicker as the particles have more kinetic energy to overcome various obstacles that they may encounter in their journey to the active site.
Concentration gradient
During osmosis and diffusion, particles move across a concentration gradient. The concentration gradient affects the rate of substances into and out of cells because the greater the difference in concentration, the greater the rate of diffusion.



