In this post
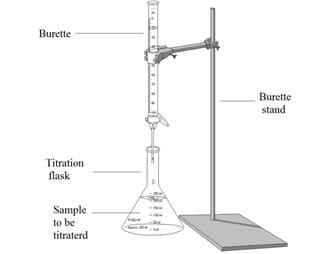
A titration is a practical technique used to determine the concentration of an acid or an alkali. In a titration, an acid or alkali with a known concentration is continually added to a sample of an acid or alkali with an unknown concentration, until the point of neutralisation is reached. The volume of the acid or alkali with a known concentration which was needed to reach neutralisation can be used to work out the concentration of the unknown solution. You must be able to describe how to carry out an acid-base titration. During a titration the equipment should be set up as shown in the diagram below:
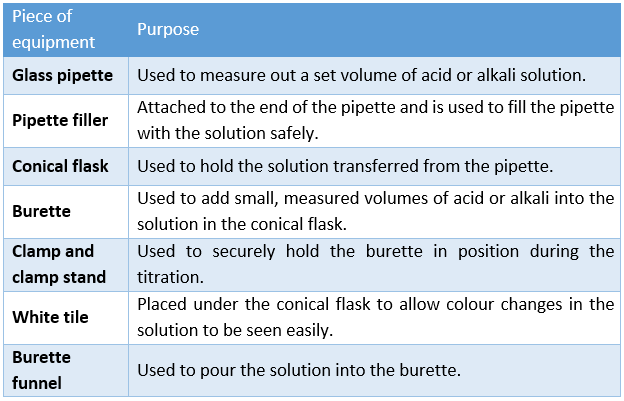
The equipment used in a titration, and their purpose, is outlined in the table below:
The method used for a titration involves the same general steps. The placing of the acid or alkali solution in the burette or conical flask is determined by the identity of the solution with the unknown solution. The solution with the known concentration is always placed in the burette and 25cm3 of the solution of unknown concentration is placed in the conical flask.
A few drops of an indicator solution such as phenolphthalein or methyl orange are added to the solution in the conical flask. These indicators will provide a single, sharp colour change as soon as neutralisation has occurred and the pH of the solution is 7.0. Universal indicator is not a suitable indicator for use in titrations as it only provides a gradual change in colour as the pH of the solution changes. The aim of the titration is to observe the end-point of the reaction which can only be indicated as a single, sharp colour change.
This point of neutralisation is known as the end-point of the reaction and the point at which no further solution should be added from the burette. This is also the finishing point of the titration and the point at which the volume of solution added from the burette is recorded. This volume can then be used in calculations to work out the concentration of the solution in the conical flask.
The method for the acid-alkali titration will now be outlined assuming that we are trying to find out the concentration of a solution of sodium hydroxide using a solution of hydrochloric acid with a known concentration.
Titration method
1. Transfer 25cm3 of the sodium hydroxide solution into a conical flask using the pipette and pipette filler.
2. Add a few drops of a suitable indicator solution such as phenolphthalein or methyl orange.
3. Fill the burette with hydrochloric acid using the burette funnel placed in the top of the burette. Make sure that when you are filling the burette the tap on the burette is closed and the burette is placed at eye level.
4. Place the conical flask containing the sodium hydroxide and indicator on a white tile below the burette.
5. Record the initial volume of hydrochloric acid in the burette. Always record your burette readings to two decimal places and remember the scale goes from 0.00 at the top of the burette to 50.00 at the bottom of the burette.
6. Before you start titrating make sure that there are no air bubbles below the tap of the burette as this would make your volume readings inaccurate.
7. Open the tap on the burette and add the hydrochloric acid into the conical flask. You should make sure that you continually swirl the conical flask to mix the solutions thoroughly.
8. As soon as the indicator changes colour, close the tap on the burette and record the final volume from the burette. Phenolphthalein is pink in alkaline solutions and colourless in neutral solutions. In this titration therefore, the colour change seen at the end-point would be pink to colourless. Methyl orange is yellow in alkali and orange in neutral solutions. For this titration the expected colour change seen at the end-point would be yellow to orange. If the solution turns red, this indicates that the solution has become acidic as too much hydrochloric acid has been added and the end-point of the reaction has been missed.
9. The first titration is known as the rough titration and is used to get a rough idea of the volume needed to reach the end-point of the reaction. The titration should be repeated again but this time the tap on the burette should be turned slightly to slow down the rate of acid solution flow to drop by drop, as the end-point volume is approached. This increases the accuracy of your titration results.
10. The volume of hydrochloric acid used is known as the titre value. It can be calculated by subtracting the initial burette reading from the final burette reading. The titration should be repeated until you achieve three titre values which are concordant. This means three titre values which are within 0.1cm3 (or 0.2cm3) of each other.
The volume of hydrochloric acid needed to neutralise the sodium hydroxide is then used to calculate the concentration of the sodium hydroxide, as shown in the following worked example.
Example – processing titration results
In a titration of 27.5cm3, 0.2 moldm-3 of hydrochloric acid was required to neutralise 25.0cm3 of sodium hydroxide solution. Calculate the concentration of the sodium hydroxide solution.
Step 1 – write the balanced symbol equation for the neutralisation reaction.
![Rendered by QuickLaTeX.com \[HCl + NaOH \rightarrow NaCl + H_2O\]](https://env-onlinelearningcollege-gpclone.kinsta.cloud/wp-content/ql-cache/quicklatex.com-0cb0809c9fccbdb5ebc3a2b9aa357c49_l3.png)
Step 2 – convert all the volumes used from cm3 into dm3.
There are 1000cm3 in 1 dm3. To convert cm3 into dm3 the value must be divided by 1000:
Step 3 – calculate the number of moles of the substance where the volume and concentration are known. To calculate the number of moles, we use the equation:
![Rendered by QuickLaTeX.com \[ \text{Number of moles} = \text{concentration} \times \text{volume} \]](https://env-onlinelearningcollege-gpclone.kinsta.cloud/wp-content/ql-cache/quicklatex.com-c95305a8ed2aa90909f996e1ad47865c_l3.png)
In this case, the volume and concentration of the hydrochloric acid are known so the number of moles can be calculated as:
![Rendered by QuickLaTeX.com \[0.2mol/dm^3 \times 0.0275dm^3 = 0.0055mol\]](https://env-onlinelearningcollege-gpclone.kinsta.cloud/wp-content/ql-cache/quicklatex.com-78133d41de7e785ba00628ccf4d20d39_l3.png)
Step 4 – calculate the unknown concentration.
To calculate the concentration of the unknown solution, we rearrange the equation used in step 3 to give us:
![Rendered by QuickLaTeX.com \[\text{concentration} = \frac{\text{number of moles}}{\text{volume of solution}}\]](https://env-onlinelearningcollege-gpclone.kinsta.cloud/wp-content/ql-cache/quicklatex.com-6f3007e048361e3a9e3f6b656e0e6363_l3.png)
We know the volume of sodium hydroxide solution used (0.025dm3). To work out the number of moles of sodium hydroxide, we use the balanced symbol equation for the reaction and the number of moles of hydrochloric acid calculated in step 3.
In the balanced equation for the reaction from step 1 we can see that sodium hydroxide and hydrochloric acid react on a 1:1 ratio. For every one mole of hydrochloric acid that reacts, one mole of sodium hydroxide reacts. We calculated that 0.0055mol of hydrochloric acid reacted so this means that 0.0055mol of sodium hydroxide reacted.
The concentration of sodium hydroxide can therefore be calculated as:
![Rendered by QuickLaTeX.com \[\text{concentration of sodium hydroxide}= \frac{0.0055mol}{0.025dm^3}\]](https://env-onlinelearningcollege-gpclone.kinsta.cloud/wp-content/ql-cache/quicklatex.com-9e4d31d516ae3fa055240ad8c59396ce_l3.png)
![Rendered by QuickLaTeX.com \[\text{concentration of sodium hydroxide}= 0.22mol/dm^3\]](https://env-onlinelearningcollege-gpclone.kinsta.cloud/wp-content/ql-cache/quicklatex.com-8af322f0c32a71bddb5945ddcf3e1718_l3.png)